Hydrogen Embrittlement
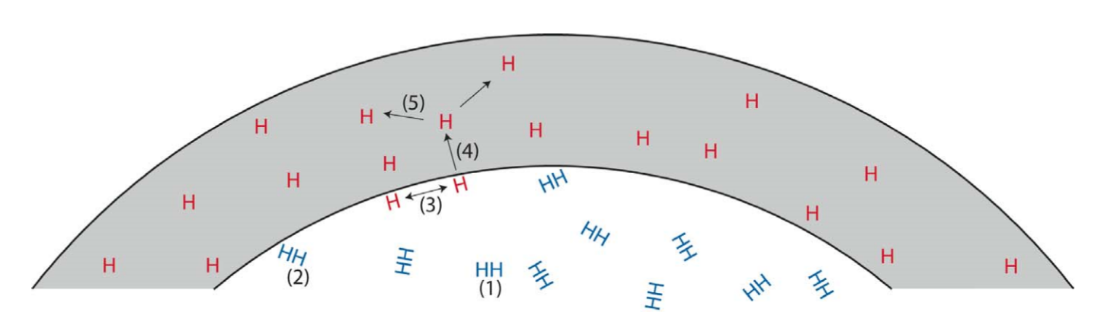
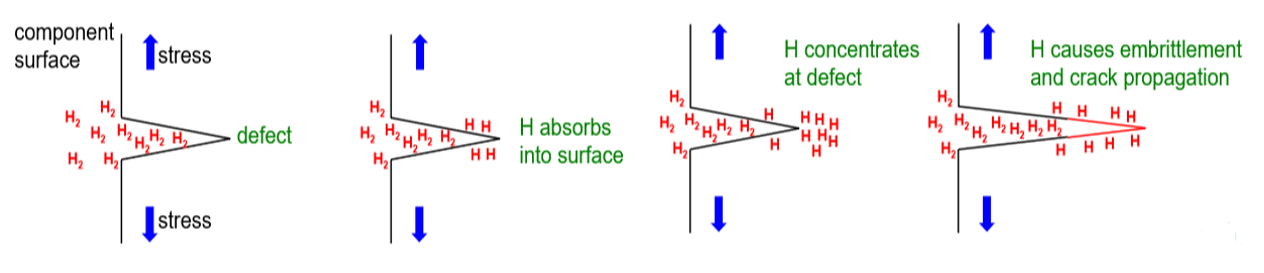
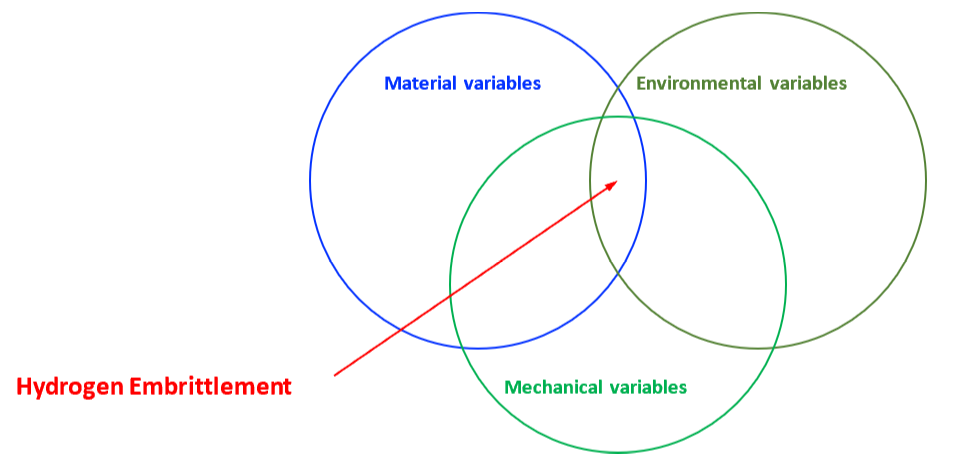
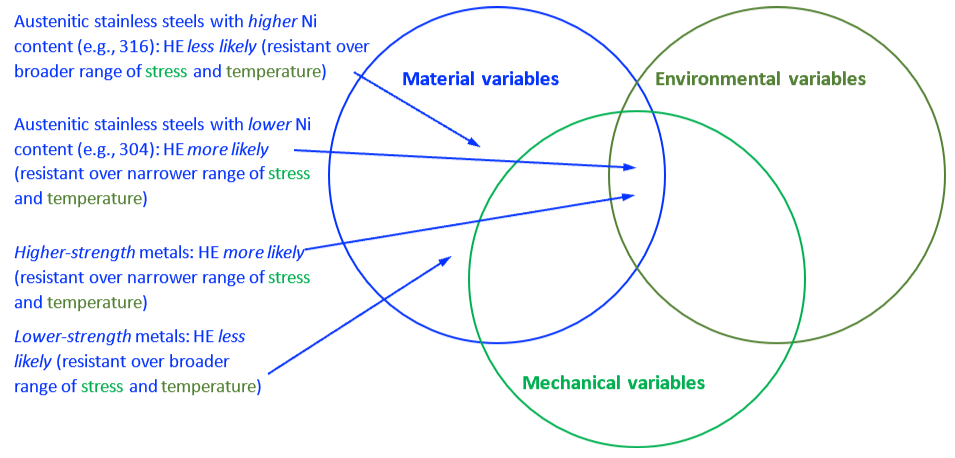
The mechanical properties of all metals are detrimentally affected by hydrogen. The magnitude of the deterioration depends on the type of metal, properties of the specific metal (e.g., strength), the environment (e.g., hydrogen pressure and temperature), and the mechanical loading. Exposure of metals to hydrogen can lead to embrittlement, which can be manifested as significant losses in tensile strength, ductility, and fracture toughness as well as accelerated fatigue crack growth. This can result in failure of pressure containing components.
An example of the effects of hydrogen-enhanced decohesion: Ruptured hydrogen cylinder and etched cross section image showing evidence of “intergranular” cracking resulting from hydrogen exposure.
References
Technical Reference For Hydrogen Compatibility of Materials - Sandia National Laboratory
Gaseous Hydrogen Embrittlement of Materials in Energy Technologies, Vol. 1 and 2, R.P. Gangloff and B.P. Somerday, Eds., Woodhead Publishing Limited, Cambridge, 2012.